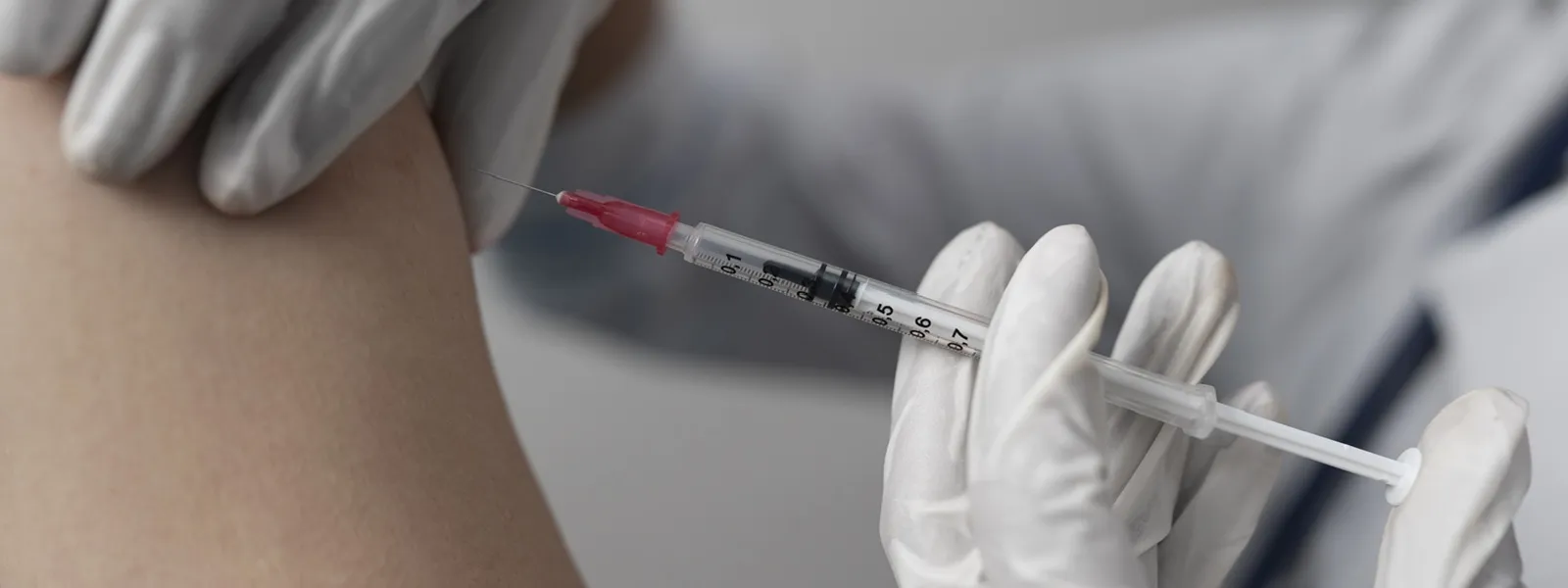
The NHS in England and Wales has approved a groundbreaking HIV prevention injection that could transform the fight against the virus. Known as cabotegravir (brand name Apretude), this long-acting injection offers protection against HIV for up to two months per dose.
Health experts have hailed the approval as a milestone in preventive medicine. Previously, daily oral PrEP tablets were the primary option for those at risk. Cabotegravir provides a discreet, low-maintenance alternative, expected to improve adherence and reduce new infections.
Trials have shown cabotegravir to be more effective than daily PrEP tablets. It works by blocking the virus from replicating in the body’s cells. Administered via intramuscular injection, it can be given at sexual health clinics and select GP practices.
“This new treatment will be life-changing for many,” said Dr. Gillian Dean, consultant in sexual health at Brighton and Sussex University Hospitals. “It removes barriers such as daily medication routines and stigma, helping more people stay protected.”
The rollout will begin in 2026 through NHS sexual health services. Priority groups include men who have sex with men, transgender individuals, and others at high risk of HIV transmission.
This approval marks a major leap in HIV prevention. Beyond its clinical value, cabotegravir represents empowerment — giving people greater control over their sexual health. As access expands, education and awareness will be essential to ensure those most in need can benefit.